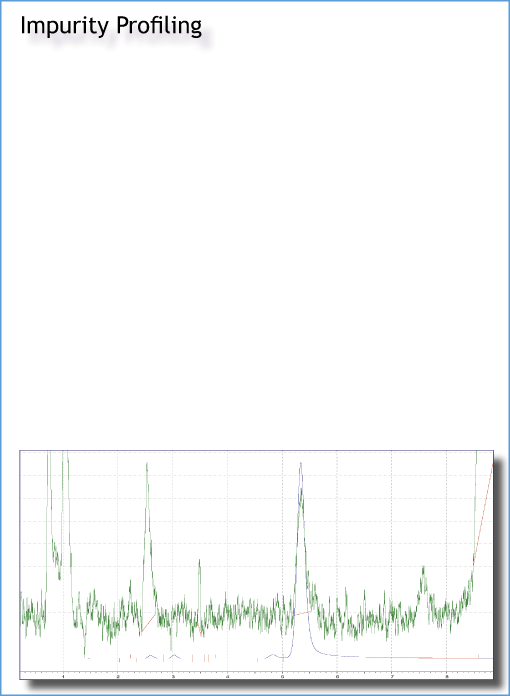

Organic impurities identified from:
- reaction mixture of a manufacturing process
- active drug material
- formulated drug preparation
The steps may involve chemical process resynthesis, elaboration of isolation methods, full structure elucidation - including IR, multinuclear NMR, LCMS, X-ray, structure proof with independent synthesis and final chromatographic confirmation from customer.
Formulated drug, immediate or delayed release as low as 1mg/tablet with impurity of 0.1A% API is typical.
Required amount synthesised impurity is delivered to the customer with purity min. 95A%.
